

In addition, GET3 has been shown to promote the targeting of short precursor polypeptides with a cleavable N-terminal SP. This implies that GET3 is involved in the targeting of tail-anchored (TA) proteins, substrates that carry a single C-terminally located TMH as a targeting signal. GET (guided entry of tail-anchored proteins) is the acronym for guided entry of tail-anchored proteins. These features prevent efficient recognition by the co-translational targeting factor SRP and require the presence of a post-translational targeting factor such as GET3. Key features of post-translationally targeted polypeptides can be a short overall precursor length (<100 amino acids), a C-terminal positioning of a transmembrane helix (TMH) that serves as a targeting signal, or, as is the case for yeast, a “weak” N-terminal signal peptide (SP) that is required for targeting. In contrast, post-translational protein targeting occurs after a precursor is fully synthesized and released from the ribosome into the cytosol. The signal recognition particle (SRP) was the first co-translationally acting targeting factor that was discovered and shown to target the ribosome-nascent chain complex (RNC) with the help of the cognate SRP receptor (SR) to the ER membrane. As soon as a specific amino acid stretch of the precursor, a so-called targeting signal, emerges from the ribosomal exit tunnel, it is recognized by a targeting factor that directs the complex of ribosome and nascent chain to the ER membrane. In the case of co-translational protein targeting, a nascent polypeptide is recognized during the process of ribosomal translation. Instead, precursor polypeptides rely on specialized targeting mechanisms that direct them to the ER membrane ( Figure 1) in a co- or post-translational fashion. Although this organelle represents a vast network that spans from the outer nuclear membrane to the periphery, newly synthesized proteins do not find the ER autonomously. Those polypeptides belong to soluble, membrane-associated, or integral membrane proteins that are all handled and distributed by the ER.
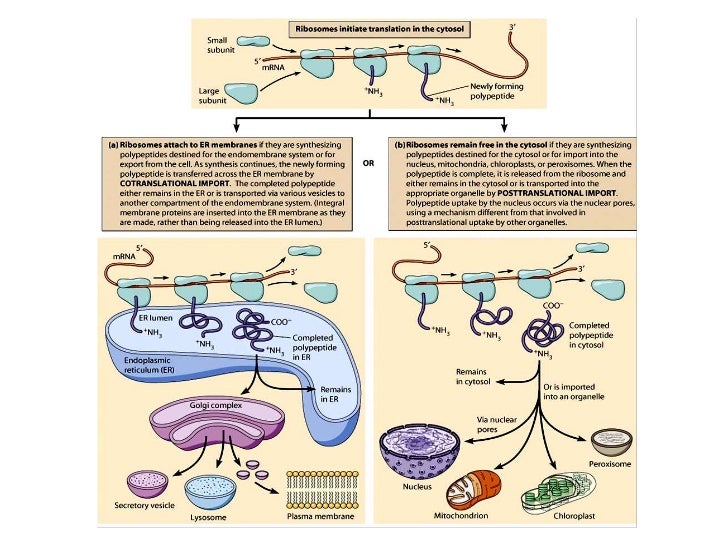

Indeed, the ER represents the entry point for proteins to the secretory pathway including the exocrine zymogens and endocrine hormones released by the pancreas.Ībout one-third of eucaryotic genes encode for polypeptides that require targeting to the ER membrane. The correlation between protein secretion and abundance of the ER is not coincidental and substantiates the importance of the ER for this process. Next to the nucleus, the ER is one of the largest organelles in many cell types and is abundantly present in secretory cells such as those found in the endo- and exocrine portions of the pancreas. In mammalian cells, the ER lumen still reflects its extracellular derivation based on the high concentration and storage of calcium. Irrespective of its inside-out (protrusions of the procaryotic plasma membrane) or outside-in (invagination of the procaryotic plasma membrane) origin, the lumen of the endoplasmic reticulum (ER) was at first similar to the extracellular milieu and therefore different from the cytosol. Different subcellular compartments have occurred during evolution as the result of either endosymbiosis, invagination of the plasma membrane, budding off from other previously formed organelles, or, as discussed more recently, from the autogenous fusion of plasma membrane protrusions. Eucaryotic cells use the principle of compartmentalization to streamline the flow of information within the crowded intracellular environment.


 0 kommentar(er)
0 kommentar(er)
